Advancing Surgical Education: Embracing Ethical and Effective Animal-Free Training Models
Written by Donya Mand
March 2024
 ©️ iStock.com/eyesfoto
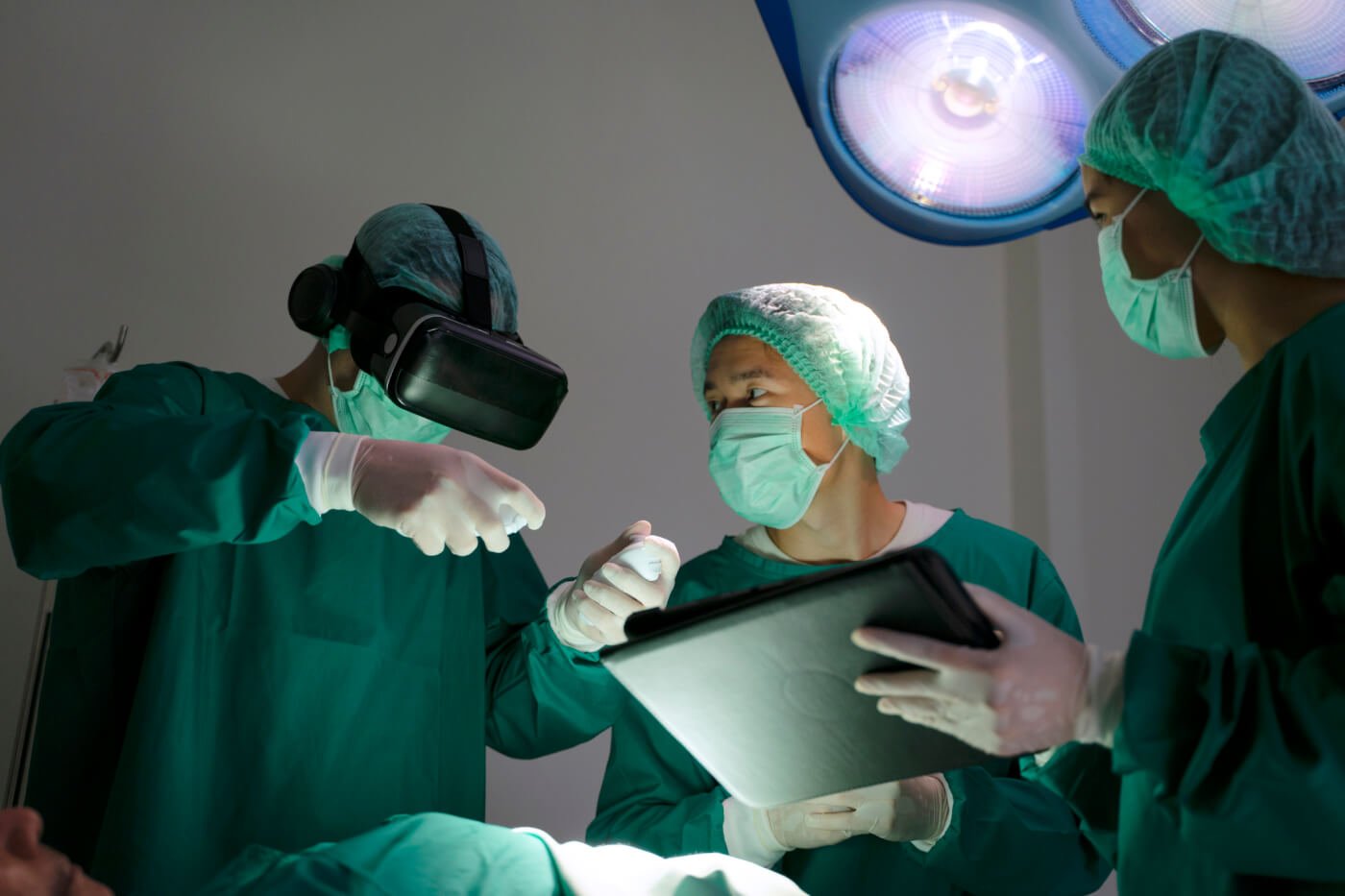
©️ iStock.com/eyesfotoThe erroneous but entrenched belief that animal models are acceptable and adequate for teaching surgical skills is one of the greatest barriers to transitioning away from using them in medical training. The goal of surgical simulation training is to use the most human-relevant models available to provide trainees with the optimal opportunity to transfer acquired skills to the operating room. Surgical educators must implement approved curricula tailored to trainees’ need to be up to date on research focusing on alternative simulation models (e.g., task trainees, 3-D printed models, synthetic organs, and virtual and augmented reality). It’s crucial that these educators regularly reassess the methods implemented at their institutions to ensure their relevance and alignment with current ethical standards.
Over the past half-century, there has been a major shift away from surgical training on live animals. Many programs now use exclusively non-animal methods (NAM) to train the next generation of surgeons. Ongoing surveys reveal that 97% of emergency medicine residency programs in the U.S. and Canada,1 85% of U.S. obstetric and gynecological programs,2 and 80% of U.S. general surgery residency programs3 no longer use live animals for training. This change is driven in part by the desire of surgical educators to help expand the existence and availability of advanced surgical simulation models and develop NAMs at their institutions, moving away from relying on misguided methods.
Surgical educators must identify a model or a combination of models in order to teach crucial technical and nontechnical operating room skills effectively. It’s imperative that these models meet ethical standards, be cost effective, and be feasible in terms of labor and setup requirements. Additionally, they should demonstrate face, content, and construct validity. As the landscape of surgical training models continuously evolves, programs must regularly reevaluate their curricula in order to provide their resident physicians and fellows with increasingly more realistic, less wasteful simulations that offer greater feedback.
For example, in the 1970s Dr. Robert Acland developed a microsurgery training course at the University of Louisville that uses live rats,4 and it still exists as a standard curriculum in the field despite substantial advances in medicine. This calls for a critical examination of the underlying rationale for using a surgical education model developed 50 years ago and raises the question of whether surgical educators have chosen the convenience of adhering to an established protocol instead of looking for a better, modern one. Perhaps this enduring reliance on animal models comes from the influence of confirmation bias, wherein surgeons trained using live animals may perceive traditional methodologies as superior due to their historical role in training surgeons, including themselves.
The use of live animals is still prevalent in microsurgical and urological training, and its stalwarts argue that it provides pulsatile arterial flow, a feature not previously found in other training methods.5 Other reasons cited include animals’ ability to replicate physiological processes, the feel of real—albeit nonhuman—tissue, the guidance of an instructor during simulation, and the development of nontechnical skills like decision-making and stress management.6
However, several programs around the world have chosen to move away from this archaic model, opting instead for more ethical, reusable, cost-effective, and equally valid or superior alternatives. Virtual reality and augmented reality simulators have now been integrated into many programs so that trainees can practice nontechnical skills such as communication and stress management.6 Other institutions, including the University of Zurich, have adapted their microsurgery curricula to use human placentas with vessels attached to a pulsatile flow system to mimic blood flow during microsurgery procedures.7
West Virginia University has developed an innovative approach that allows urology trainees to practice on perfused fresh human cadavers, providing experience with anatomically relevant human tissues and organs.8 The University of Rochester’s use of 3-D printing technology has enabled the creation of phantom models for complex procedures, such as prostatectomy, replicating the mechanical properties of human organs more closely than traditional animal model constructs.9 The staff there uses “PVA hydrogel that increases stiffness with each successive freeze-thaw cycle to mimic the mechanical properties of the prostate gland …. Once removed from the corresponding moulds, the organs were cast in a series: prostate, seminal vesicle, neurovascular bundle, bladder and urethra moulds to replicate anatomical relationships between various organs …. The final synthetic hydrogel organ model system closely replicates the human organ model system. This is in contrast to animal model constructs, which do not replicate this male genitourinary system.”10
Educators must choose either to rely on misguided methodologies or to innovate and lead the field of surgical education. Surgical instructors and medical institutions should be obligated to explore the latest NAMs to ensure that their surgical training programs are compassionate, ethically aligned with contemporary standards, and using the most advanced tools available to serve the needs of a new generation of surgeons.
1Physicians Committee for Responsible Medicine. Survey of Emergency Medicine Residencies. Updated October 17, 2023. Accessed February 26, 2023. https://www.pcrm.org/ethical-science/ethical-education-and-training/survey-of-emergency-medicine-residencies
2People for the Ethical Treatment of Animals. Animal Use in OB/GYN Physician Residency Training Programs in the U.S. Updated February 15, 2024. Accessed February 26, 2024. https://www.peta.org/action/animal-use-in-ob-gyn-physician-residency-training-programs/
3Physicians Committee for Responsible Medicine. Survey of Surgery Residencies. Updated October 17, 2023. Accessed February 26, 2023. https://www.pcrm.org/ethical-science/ethical-education-and-training/survey-of-surgery-residencies
4Perez-Abadia G, Pindur L, Frank J, et al. Intensive Hands-On Microsurgery Course Provides a Solid Foundation for Performing Clinical Microvascular Surgery. Eur J Trauma Emerg Surg. 2023;49(1):115-123. doi:10.1007/s00068-022-02040-8
5Gasteratos K, Paladino JR, Akelina Y, Mayer HF. Superiority of Living Animal Models in Microsurgical Training: Beyond Technical Expertise. Eur J Plast Surg. 2021;44(2):167-176. doi:10.1007/s00238-021-01798-1
6Nassar AK, Al-Manaseer F, Knowlton LM, Tuma F. Virtual Reality (VR) as a Simulation Modality for Technical Skills Acquisition. Ann Med Surg (Lond). 2021;71:102945. doi:10.1016/j.amsu.2021.102945
7Höbner LM, Staartjes VE, Colombo E, Sebök M, Regli L, Esposito G. How We Do It: The Zurich Microsurgery Lab technique for placenta preparation. Acta Neurochir (Wien). 2023;165(12):3821-3824. doi:10.1007/s00701-023-05847-5
8McClelland D, O’Connor LP, Barnard J, et al. The Utilization of Perfused Cadaver Simulation in Urologic Training: A Pilot Study. BMC Urol. 2021;21(1):134. doi:10.1186/s12894-021-00895-4
9Witthaus MW, Farooq S, Melnyk R, et al. Incorporation and Validation of Clinically Relevant Performance Metrics of Simulation (CRPMS) Into a Novel Full-Immersion Simulation Platform for Nerve-Sparing Robot-Assisted Radical Prostatectomy (NS-RARP) Utilizing Three-Dimensional Printing and Hydrogel Casting Technology. BJU Int. 2020;125(2):322-332. doi:10.1111/bju.14940
10Costello DM, Huntington I, Burke G, et al. A Review of Simulation Training and New 3D Computer-Generated Synthetic Organs for Robotic Surgery Education. J Robot Surg. 2022;16(4):749-763. doi:10.1007/s11701-021-01302-8
